Artisan organic soap and cosmetic manufacturers are very meticulous about their ingredients and packaging material, but when it comes to one of the simplest and most common ingredients they are confronted with a big puzzle and confusing information.
Unless you’re making exclusively anhydrous products such as oil blends or balms and butters, the highest concentration ingredient in many cosmetic products (and generally the first on most INCI ingredients lists) is water (aqua).
No matter if you’re using plain water or a hydrosol in your product, the starting material is water. So here are the two big questions we are regularly asked by organic cosmetic formulators:
- Which type of water should you use in your cosmetic formulations?
- What’s the difference between distilled and deionized water?
Tap water undergoes several physical and microbiological filtrations before it reaches us. It should be completely alright for normal household procedures (hopefully) and free of pathogenic microorganisms. For cooking and drinking purposes however, depending on the quality of the tap water in your area, you may need other step(s) of filtration to reduce its hardness, and remove suspended particles. Some sensitive household instruments may even have a built-in filter in order to prolong the instrument’s life and improve its performance.
When talking about water quality, these are the most general and important factors to be considered:
- Physical impurities and particulates
- Inorganic ions (minerals and salts)
- Organic compounds (TOC)
- Bacteria (TVC)
- Pathogens and endotoxins
Physical filtration is one of the first steps in water purification and preparation (for example by an activated charcoal filter). This step removes suspended particles, colouring material etc. from water.
A further pre-treatment is usually carried on to reduce the organic matter and the bacteria (specifically pathogenic micro-organisms).
After these pre-treatments and depending on the application, the water may be treated by:
- Distillation and double distillation
- Ion-exchange
- Demineralization
- Nano filtration
- Reverse Osmosis (RO)
Distillation of water
Distillation is one of the oldest water purification procedures. The procedure is pretty much the same as distillation of a hydrosol or an essential oil. The water is boiled and the resulting water vapour is re-condensed to water at the other end.
This is one of the most expensive and energy consuming water treatment procedures. In this process, all suspended material (if present), mineral and microbiological contaminants are left behind. However, organic and volatile material with a boiling point less than or near to that of water would be carried away with water vapour through the condenser.
For certain laboratory applications, a second distillation is carried out and this water is called bidest or double distilled water. This method is only confined to certain laboratory procedures however.
Deionization of water
Deionization is as a well-known old method for water purification. In this method, special ion-exchange resins remove anions and cations present in water with hydronium and hydroxide ions (H3O+, OH-). The result is a deionized water. Most dissolved water impurities are salts such as Magnesium sulphate, Sodium Chloride etc. Cation exchangers retain cations (Magnesium++, Calcium++, Na+, etc.) and release hydronium ion (H3O+) and anion exchangers retain anions (Sulphate, Chloride, etc.) and release hydroxide ion (OH-).
This method does not purify microorganisms, volatile material, organic impurities or suspended material. After a period of time (depending on the original quality of the water) the ion-exchange resins are saturated and are re-generated for further use.
Demineralization of water
The demineralization of water is most often mistakenly interchanged with deionization – but the outcome of these two processes is different. Demineralization can be performed with ion exchangers or other filtration systems but the main purpose is to soften the water by removing Calcium and Magnesium ions. The resulting demineralized water contains Sodium Chloride which is absent in deionized water.
Nano filtration of water
Nano filtration is regarded as a partial reverse-osmosis procedure. This procedure is mainly applied in preparation of drinking water and removes bivalent ions such as Calcium, Magnesium, Sulphate and heavy metal ions. It has only partial efficacy in removing Sodium Chloride from water.
Reverse Osmosis (RO) of water
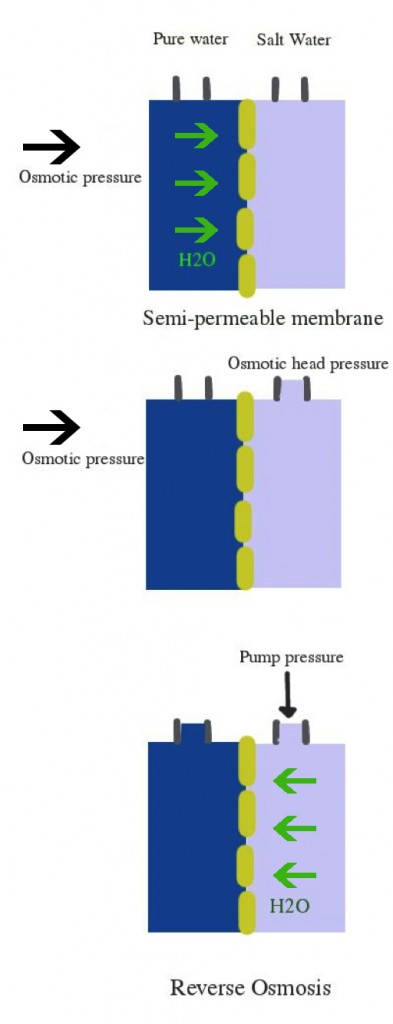
Now imagine a pressure is applied over the right compartment. This pressure would press the water through the membrane so that water migrates from the right compartment (salt water) to the left compartment, while dissolved minerals, large molecules (organic molecules), oils and bacteria are left behind.
There is however evidence that bacteria, and specifically gram negative bacteria can grow on the membrane and in RO water. It is therefore necessary to perform an extra disinfection step if you’re using RO water in your cosmetic lab.
This method is effective and affordable. It is often used as a pre-treatment in combination with other techniques. The membrane should be changed after a while. RO removes 90-99% of water impurities. The quality of water and the lifespan of the membrane depend on the quality of the feed water.
RO can be combined with other water purification methods to improve the water quality and to remove the remaining 1-10% impurities.
Which water should you use in your cosmetic lab?
When we take all factors into account, distilled water has the highest quality for a cosmetic lab but distilled water is not always achievable and affordable. As an alternative, you can use deionized or RO water in your cosmetic lab.
Best practise is to use freshly boiled water in your cosmetic lab. If you’re working on a large scale then you should consult a professional water purification company to find the best choice for your facility.
A comparison of different water treatment methods:
| Ca++, Mg++ | Sulphate | NaCl | Physical impurities, suspended particles | Organic material | Bacteria, viruses | |
| Distillation | ++ | ++ | ++ | ++ | + | ++ |
| Deionization | ++ | ++ | ++ | – | – | – |
| Demineralization | ++ | ++ | – | – | – | – |
| Physical filtration | – | – | – | ++ | – | – |
| Boiling | – | – | – | – | + | ++ |
| RO | ++ | ++ | ++ | ++ | + | + |
| Nano-filtration | ++ | ++ | – | ++ | – | – |
- ++ = very effective
- + = effective
- – = not effective
Which water do you work with in your cosmetics? Leave us a comment below.
References
http://www.fda.gov/ICECI/Inspections/InspectionGuides/InspectionTechnicalGuides/ucm072913.htm
http://www.labconco.com/news/whats-the-difference-between-ro-and-di-water-pur
http://www.lenntech.com/processes/pesticide/nanofiltration/nanofiltration.htm
FREE TRAINING
Learn how to become an
Organic Skincare Formulator
FREE TRAINING
How to become an
Organic Skincare Entrepreneur
FREE TRAINING
How to become an
Organic Skincare Entrepreneur
Leave us a comment

Dr. Elham Eghbali was Formula Botanica’s Cosmetic Chemist between 2014 and 2018. She has over 20 years’ industry experience and is based in Bavaria, Germany. To read more about Formula Botanica’s team, visit our staff page.